Written by Asmer Aliyeva
Edited by Dr. Hannah K Shorrock
Antisense Oligonucleotide (ASO) treatment improves Purkinje neuron function regulated by potassium channels and highlights altered brain connectivity in movement disruption in SCA3.
Understanding which cell types are important for disease onset is vital for therapy development and treatment options for patients. If a cell type isn’t affected, we don’t need to target it with therapies. To help our understanding of relevant cell types in SCA3, this study set out to understand the role Purkinje neuron abnormalities play in disease.
Interestingly, in SCA3 Purkinje neurons do not degenerate, or die, to the same extent as is seen in other ataxias. This could suggest a less important role of these cells in motor impairment in SCA3. To investigate if this is true, the authors asked whether antisense oligonucleotide (ASO) treatment could rescue changes in Purkinje neuron dysfunction and improve motor behavior in SCA3? The result: ASO treatment does improve Purkinje neuron dysfunction in SCA3, to a certain extent.
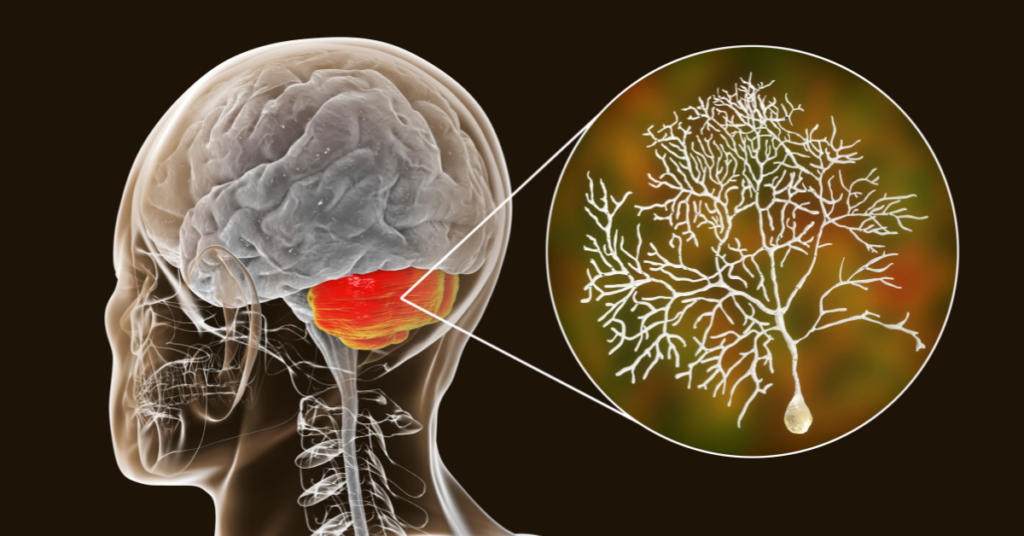
Spinocerebellar ataxia type 3 (SCA3), also known as Machado-Joseph Disease or MJD, is a dominantly inherited form of ataxia. Caused by the expansion of CAG repeats in the gene called ATXN3, this expansion results in a longer polyglutamine sequence in the ATXN3 protein. SCA3 is characterized by progressive loss of neurons in multiple areas of the brain. One of these brain regions is the cerebellum. It is known that the cerebellum controls our coordination and balance, and proper functioning of Purkinje cells is essential for the execution of these behaviors.
Healthy neurons use electrical impulses that enable neurons to communicate with each other. This process is known as neuron firing or spiking. Coordinated work of different ion channels support normal and consistent spiking in Purkinje neurons. In early stage SCA3 mouse models, Purkinje neurons lose their spontaneous spiking and there is reduced activity of certain ion channels that allow potassium ions to move in and out of the neuron.
Alterations in how these potassium ion channels function contribute to changes in Purkinje neuron function and problems with movement. However, the exact role of neuron spiking in causing movement problems is still not clear. The authors of this study asked the question: can ASO treatment rescue ion channel function, neuron firing and movement? To do this, the researchers looked at early-, mid- and late-disease stages in a SCA3 mouse model with, or without ASO treatment.
They looked at six different potassium channels at each of these disease stages in a SCA3 mouse model. At the early disease stage, one of these ion channels showed changes in the amount of the channel protein being made by the body (also called ‘expression’ by scientists). By the mid-disease stage, two of the ion channels showed changes in their expression.
For five of the channels, there were changes between unaffected and SCA3 mice at the late disease stage. Interestingly, however, the size of the changes in expression did not increase from mid to late disease stages for the channels affected at both time points. Because the amount of these potassium ion channels is changed in SCAs, this could affect Purkinje neuron function, or more specifically, Purkinje neuron spiking.
To understand if these changes in potassium ion channel expression caused changes to Purkinje neuron spiking the authors measured the activity of the neurons. In the mid-disease stage mice, in two different regions of the cerebellum, the researchers found that the neurons fired more frequently in SCA3 mice than in unaffected mice. This increased firing frequency, or increased spiking, indicates that the Purkinje neurons have increased excitability.
This hyperexcitability (increased excitability) of Purkinje neurons can be problematic. Because the changes in ion channel expression persist later into disease, the research group wanted to know if the changes in neuron excitability persist as well. The answer, yes. At the late disease stage, the researchers found that the Purkinje neurons in one of these cerebellar regions were also hyperexcitable.
Together, these data suggest that although changes in Purkinje neuron activities in older mice exist, they do not differ from the younger mice. This means that the progressive worsening of motor function that occurs in the SCA3 mice over time is not due to a progressive worsening of Purkinje neuron function. The absence of further changes in Purkinje neurons at these late disease stages might suggest that alternations to neuron function in other brain regions contribute to the progressive motor dysfunction in later disease stages in SCA3 mice.
The authors wanted to know if ASO-based reduction of ATXN3 gene expression could rescue increased neuronal activity and potassium ion channel expression levels in SCA3 mice. The mice were treated with ASO for eight weeks and their behavior, neuron function, and ion channel expression were analyzed at the same mid-stage of disease.
The untreated SCA3 mice were less active than the unaffected mice. The SCA3 mice treated with the ASO, however, showed similar levels of movement to the unaffected mice. The ASO treatment also restored the expression of the potassium ion channels and reversed the hyperexcitability. Together these data show that ASO treatment can effectively rescue impairments in motor activities at a time when potassium channel dysfunction is observed in SCA3 mice.
The goal of this study was to understand the involvement of Purkinje neurons in SCA3 and explore gene suppression strategies as potential targets for therapy development. The ASO treatment used was able to rescue the expression of several key ion channels, restore Purkinje neuron spiking frequency, and increase the movement of SCA3 mice to the levels of unaffected mice. This data using ASO treatment suggests that alternations to Purkinje neuron function contribute meaningfully to movement problems during the SCA3 disease process.
However, while this data is promising, it also raises several questions. The researchers identified that changes in Purkinje neuron function do not progress as the movement problems get worse over time during SCA3 disease. Because of this, the researchers suggest that changes in the cerebellum are not the only drivers of progressive movement problems in SCA3. They suggest that other brain regions beyond the cerebellum might be involved. So, when, and where do these brain regions become important for SCA3 disease? More research needs to be done to better understand the role not only of Purkinje neuron function in SCA3, but also to understand the contribution of different brain regions, and the interplay between these regions, to the disease process in SCA3.
Key Words
Neuronal Firing: The use of electrical impulses for communication between neurons.
Conflict of Interest Statement
The author and editor have no conflicts of interest to declare.
Citation of Article Reviewed
Bushart DD, Zalon AJ, Zhang H, Morrison LM, Guan Y, Paulson HL, Shakkottai VG, McLoughlin HS. Antisense Oligonucleotide Therapy Targeted Against ATXN3 Improves Potassium Channel-Mediated Purkinje Neuron Dysfunction in Spinocerebellar Ataxia Type 3. Cerebellum. 2021 Feb;20(1):41-53. doi: 10.1007/s12311-020-01179-7. https://pubmed.ncbi.nlm.nih.gov/32789747/
Read Other SCAsource Summary Articles
Targeting Mutant Ataxin-3 with Antisense Oligonucleotides can Improve Purkinje Neuron Dysfunction
Written by Asmer Aliyeva Edited by Dr. Hannah K Shorrock Antisense Oligonucleotide (ASO) treatment improves Purkinje neuron function regulated by potassium channels and highlights altered brain connectivity in movement disruption Read More…


Walking toward a better understanding of SCA3 progression
Written by Alexandra Putka Edited by Dr. Hayley S. McLoughlin Walking measurements detect changes in SCA3 severity across a 1-year study and represent an important biomarker of disease progression Medical Read More…


¿Ser o no ser SCA27B? Esa es la cuestión: Misteriosos casos de ataxia resueltos gracias a los avances en tecnología genética
Escrito por Dr. Hayley McLoughlin y Dr. Sharan SrinivasanEditado por Dr. Celeste SuartTraducido por Ismael Araujo-Aliaga Una nueva tecnología de secuenciación genética reveló numerosos casos de ataxia familiar asociados a Read More…









